[統計分析] 強烈建議大家第三針打復必泰
本是同林鳥
475 回覆
515 Like
797 Dislike
三針打復必泰都中左,防都冇得防
你了解咗死亡證係點寫先
https://www.facebook.com/muk.lam/posts/10158297047746496
https://www.facebook.com/muk.lam/posts/10158297047746496
最近常有人吐槽「為甚麼打疫苗後死亡與疫苗無關,但帶著covid病毒死去就是因covid造成」,在此回應。
1. 死亡證: 為甚麼帶著covid病毒死去就是因covid造成
在香港,死亡證明上要寫死因,有直接死因(a項),也有間接死因(b項及c項)。假設一位40歲無長期病患者因心臟病發而猝死,那他的直接死因就是「心臟病發」。心臟病發與死亡有時序關係,而在此案例中,我們找不到其他解釋他死亡的原因,所以心臟病發是死因。
grace kelly在楂車時心臟病發,出車禍後死亡。我不清楚詳細,假設她心臟病發時馬上心臟停頓,陳屍駕駛座上,然後出車禍,那直接死因就是心臟病發。反之,若果她心臟病發後只是失去意識或胸痛,但因車禍的外傷而死,那直接死因就是車禍。
假設一位100歲的失智老人因covid肺炎死亡,那他的直接死因是covid,間接死因則是失智/高齡。如果没有染上covid,他大概不會馬上死亡。當然,以他的年齡,死亡是自然的人生歷程,covid肺炎不過一個誘因,就算没有covid,他也會在不久的將來因其他感染過世。所以他的間接死因還應該加上失智。
這個例子中的老人是die of covid還是die with covid,是有討論空間的。covid引起各國政府重視的原因,不只是因為它攻擊老人,還因為它的確造成不少過往健康的壯年人「没有其他原因解釋」的死亡,使受covid肆虐的國家的壯年死亡率比往年上升。
香港醫院內部有不少病菌爆發,最新爆發的是Candida Auris,它是人類皮膚菌落,普通人沾上没大問題,但一旦需要長年插胃喉/尿喉的長期病患沾上,這種惡菌便有可能沿異物進入人體,造成尿道炎或細菌入血。政府醫院對這種細菌嚴陣以待,花了很多資源去圍堵它,但普羅市民幾乎没怎麼聽說過它,因為它不會影響壯年人口。
很可惜,COVID會影響壯年人口。我們都希望所有患上COVID過世的是高齡老人,是DIE WITH COVID而不是DIE OF COVID,但我們需要觀察。
Sera were randomly selected from two previously studied cohorts to investigate the immunogenicity of two doses10 or booster doses11 of COVID-19 vaccines. The first study had sera collected 3–5 weeks after the second dose of CoronaVac (28 d between the first and second dose) or BNT162b2 vaccine (21 d between first and second dose)10. The second cohort had sera collected 3–5 weeks after booster vaccination of individuals vaccinated with CoronaVac who were given a third dose of CoronaVac (the interval between the second and third vaccine dose was 61–160 d; 17 of them were also included in the previous two-dose CoronaVac study) or BNT162b2 (interval between the second and third vaccine dose was 51–141 d; 9 of them were also included in the previous two-dose CoronaVac study)11. A third group consisted of individuals vaccinated with BNT162b2 receiving a third dose of BNT162b2 (180–234 d after the second dose; 4 of them were included in the previous two-dose BNT162b2 study) with serum collected 3–5 weeks after the third vaccine dose. None of these individuals had a previous history of SARS-CoV-2 infection confirmed with negative IgG to SARS-CoV-2 receptor-binding domain antibody15 in the prefirst vaccine dose serum.
其實正常Paper嘅Methodology一定有寫
不過有冇人睇完先9up係另一回事
source:
https://www.nature.com/articles/s41591-022-01704-7#Sec2
其實正常Paper嘅Methodology一定有寫
不過有冇人睇完先9up係另一回事

source:
https://www.nature.com/articles/s41591-022-01704-7#Sec2
香港老人家接種率特別低
而且仲要打科興
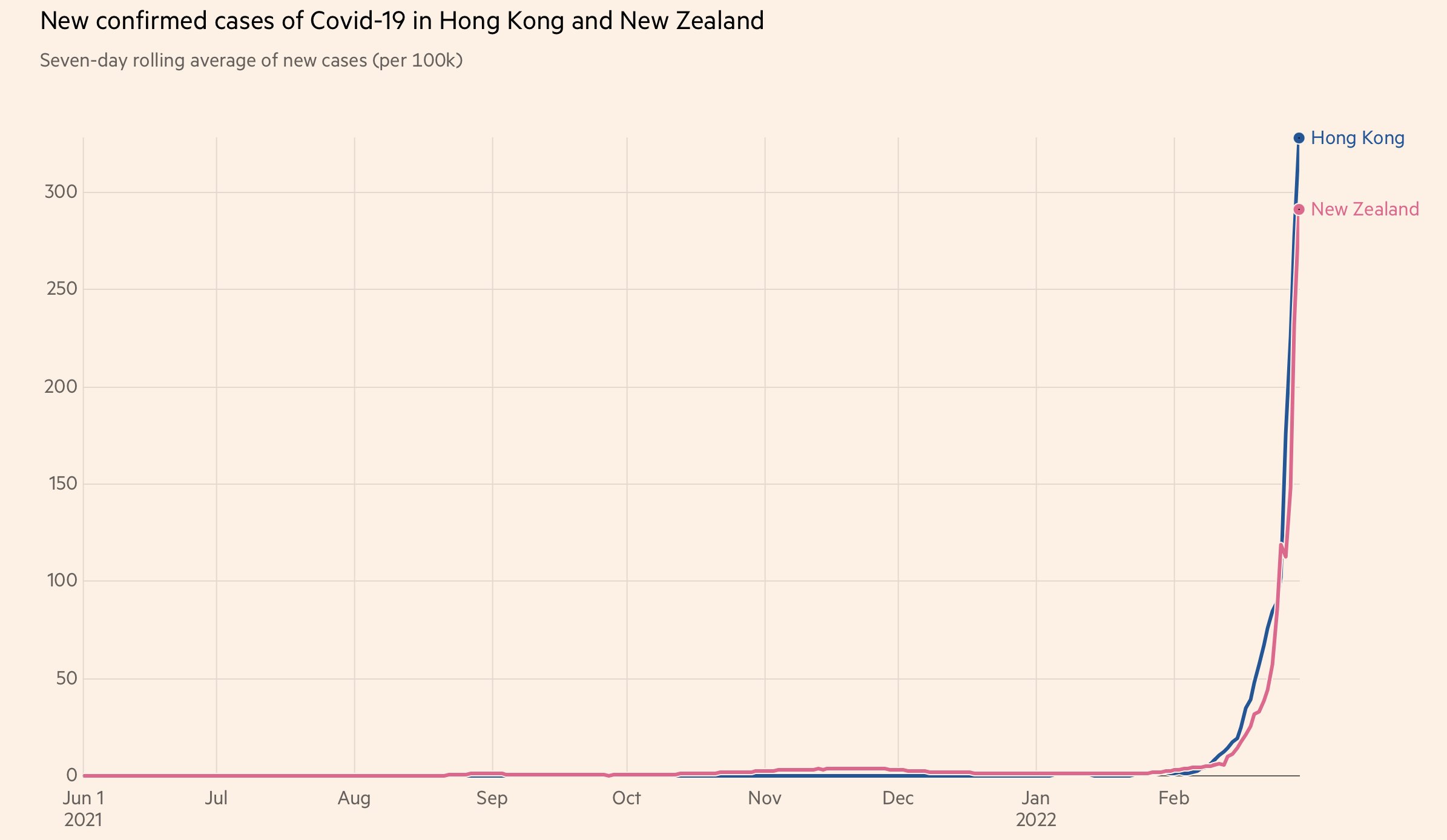
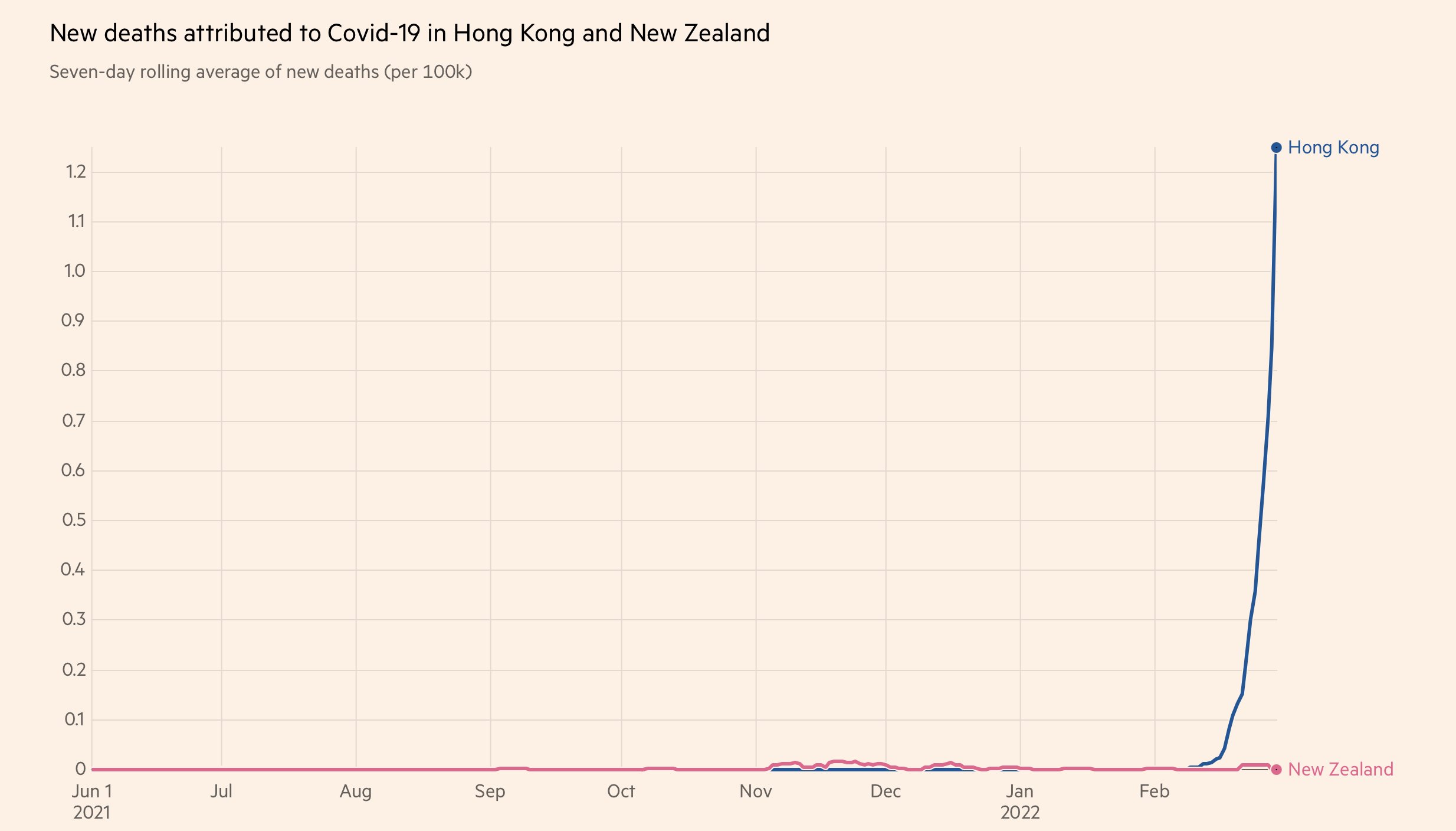
紐西蘭嘅情況同香港情況類似,都係長期零確診然後近排爆
但係點解人地死亡率基本上無上升
因爲人地老人家打晒針
https://twitter.com/InfectiousDz/status/1499091943044919302
而且仲要打科興
紐西蘭嘅情況同香港情況類似,都係長期零確診然後近排爆
但係點解人地死亡率基本上無上升
因爲人地老人家打晒針
https://twitter.com/InfectiousDz/status/1499091943044919302


Hong Kong has more who are elderly and fewer who are vaccinated than in New Zealand. It's the elderly who die from COVID. As Omicron spread in HK, vaccine hesitancy has led to vaccination rates among those aged 70-79 and 80-plus to be just 63.1% and 34.2%
https://cnbc.com/2022/02/10/hong-kong-vaccination-rates-spike-as-outbreak-shatters-covid-free-dreams.html
Meanwhile in New Zealand, vaccination rates in the elderly are over 95% https://www.health.govt.nz/covid-19-novel-coronavirus/covid-19-data-and-statistics/covid-19-vaccine-data
3~5 weeks 姐係同第一二支一樣啦 過多幾個月咪又係drop 

指第三針嘅感覺定中肺炎?
第三針就手臂痛only
中肺炎就第一日發燒37.7度,之後冇燒過
鼻塞流鼻水,咳,有痰
有兩日個味覺變得好淡
冇喉嚨痛
第三針就手臂痛only
中肺炎就第一日發燒37.7度,之後冇燒過
鼻塞流鼻水,咳,有痰
有兩日個味覺變得好淡
冇喉嚨痛

Political Science? 
 信,我一定信呀
信,我一定信呀

 信,我一定信呀
信,我一定信呀可以長生不老
最勁係一個月打一支
佢問你打完三針都中係咩感覺
升得快,跌得快,睇嚟要一個月打一針先可以keep 到夠抗體

Drop 係正常表現
Even 而家有 study 證明打第4針都唔會明顯提高對Omicron嘅efficacy
source:
https://www.nature.com/articles/d41586-022-00486-9
https://www.medrxiv.org/content/10.1101/2022.02.15.22270948v1.full-text
Even 而家有 study 證明打第4針都唔會明顯提高對Omicron嘅efficacy

source:
https://www.nature.com/articles/d41586-022-00486-9
https://www.medrxiv.org/content/10.1101/2022.02.15.22270948v1.full-text
我3針BNT後都中左
全家都中, 但我病徵最輕連燒都無
全家都中, 但我病徵最輕連燒都無
你唔好忘記有啲香港長者打4hing,
第二係人地醫院唔會全部陽性個案都收,
淨係收重症,
唔似垃圾港共輕症都收入院,無人照顧下變嚴重
第三係外國醫院無呢堆野外tents,
個個有需要入院嘅都正常收入院,上病房密切照顧
你咁compare喺omit咗太多野,
根本人禍俾你完全omit晒
第二係人地醫院唔會全部陽性個案都收,
淨係收重症,
唔似垃圾港共輕症都收入院,無人照顧下變嚴重
第三係外國醫院無呢堆野外tents,
個個有需要入院嘅都正常收入院,上病房密切照顧
你咁compare喺omit咗太多野,
根本人禍俾你完全omit晒
打兩針都未見到zoom仔仲叫我打第三針?
你老尾自己打飽佢啦
你老尾自己打飽佢啦

咁咪即係無否認我講嘅野囉
打針就全部die with打針,唔係die of打針
陽性死亡個案就全部die of covid,唔係die with covid
根本你同政府一樣,
雙重標準
講真,好多陽性個案但輕微嘅老人家
如果唔收入院,由返院舍同家人照顧,
根本就唔駛死
有一部分人惡化變嚴重case
反而係因為入院
因為啲護士真係做到1:25根本無人手睇
你咁搞法,本身輕症入去都惡化到變重症啦
打針就全部die with打針,唔係die of打針
陽性死亡個案就全部die of covid,唔係die with covid
根本你同政府一樣,
雙重標準

講真,好多陽性個案但輕微嘅老人家
如果唔收入院,由返院舍同家人照顧,
根本就唔駛死
有一部分人惡化變嚴重case
反而係因為入院
因為啲護士真係做到1:25根本無人手睇
你咁搞法,本身輕症入去都惡化到變重症啦
變xmen
會,有機會有cytokine storm
點樣無否認?
打完針即刻死噉當然係die of vaccine啦
插住呼吸機噉死當然係die of covid㗎,呢個咪係「直接死因」囉
點樣雙重標準?
就算係喺外國,唔會強制要老人家等入院,老人死亡率都係好高㗎啵
打完針即刻死噉當然係die of vaccine啦
插住呼吸機噉死當然係die of covid㗎,呢個咪係「直接死因」囉
點樣雙重標準?
就算係喺外國,唔會強制要老人家等入院,老人死亡率都係好高㗎啵
不嬲都唔應該打科興架啦
不過呢度係連登
五毛, 藍絲, 奶共, PR, 苗撚, 恐懼撚, 左膠, 樓主要邊個?
不過呢度係連登
五毛, 藍絲, 奶共, PR, 苗撚, 恐懼撚, 左膠, 樓主要邊個?
問題係打針誘發咗啲本來唔會咁快病發嘅心血管疾病
中風,爆腦血管瘤
咁又唔係打完針即刻死,所以又唔計啦

插住呼吸機?
你知唔知好多老人本身有COPD,
或者吸緊氧氣,比你放喺戶外8度9度吹冷風,
搞到氣管收縮惡化
咁又計埋入Covid數


屌同你講都晒氣
中風,爆腦血管瘤
咁又唔係打完針即刻死,所以又唔計啦


插住呼吸機?
你知唔知好多老人本身有COPD,
或者吸緊氧氣,比你放喺戶外8度9度吹冷風,
搞到氣管收縮惡化
咁又計埋入Covid數



屌同你講都晒氣
唔同長期病患的人打完之後抗體水平
呢種白老鼠好難搵
Base on現階段
都係比較偏向你自己個人願意去信定唔願意去信
呢種白老鼠好難搵

Base on現階段
都係比較偏向你自己個人願意去信定唔願意去信