毒L 藥劑師,你問我答
藥劑連缐
1001 回覆
176 Like
17 Dislike
Lm dispenser 路過求學野
香港邊到有實驗室用具買?
濕疹有邊隻潤膚膏推介?好返既濕疹有黑色素沉澱可以點搞?
點睇李家誠個隻抗衰老藥,樂加欣。你覺得有冇效
Polaprezinc (Zinc L-carnosine) 用係胃炎到係一個相對地少研究的藥物。我只搵到中國同南韓有兩份paper係話佢對於 H. poly 引起的慢性胃炎有效。以我查到的資料而言,我會認為現時無確切證據說明其成效。
加拿大視佢為實驗性質
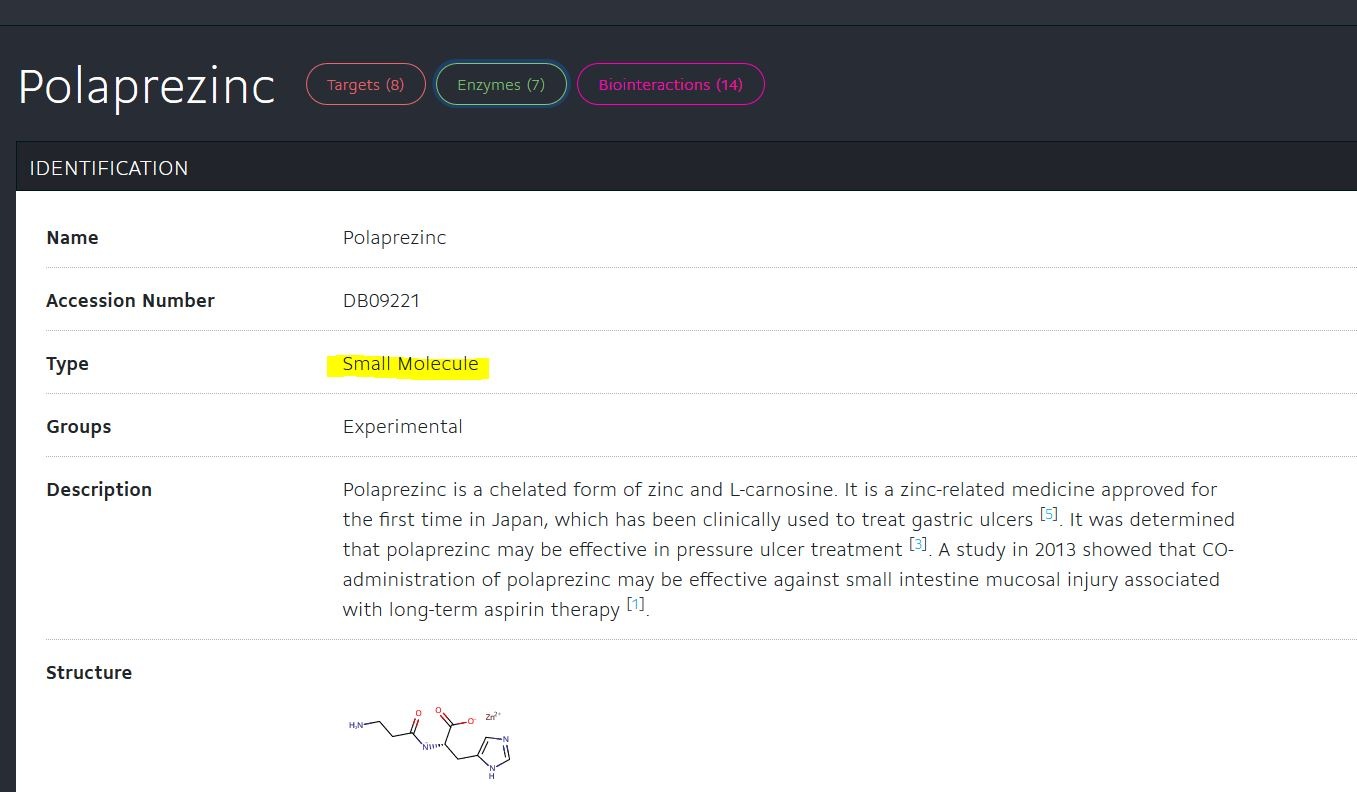
https://www.drugbank.ca/drugs/DB09221
澳洲視佢為輔助性藥物(complementary)
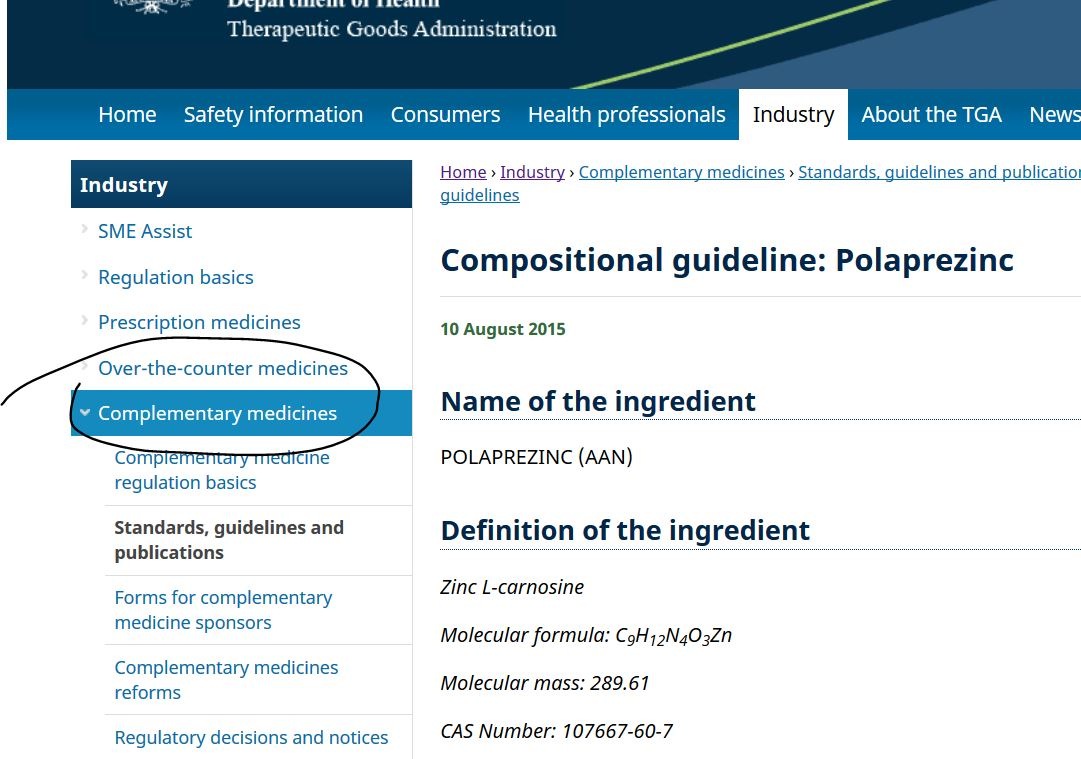
https://www.tga.gov.au/compositional-guideline/polaprezinc
係我手上幾個references都無講太多
MIMs Online: No Product Information (PI) 同 Consumer Medicines Information (CMI)

Australian Medicine Handbook: Not found
Micromedex:
Cochrane Library: 只有一個entry, 但係講radiotherapy
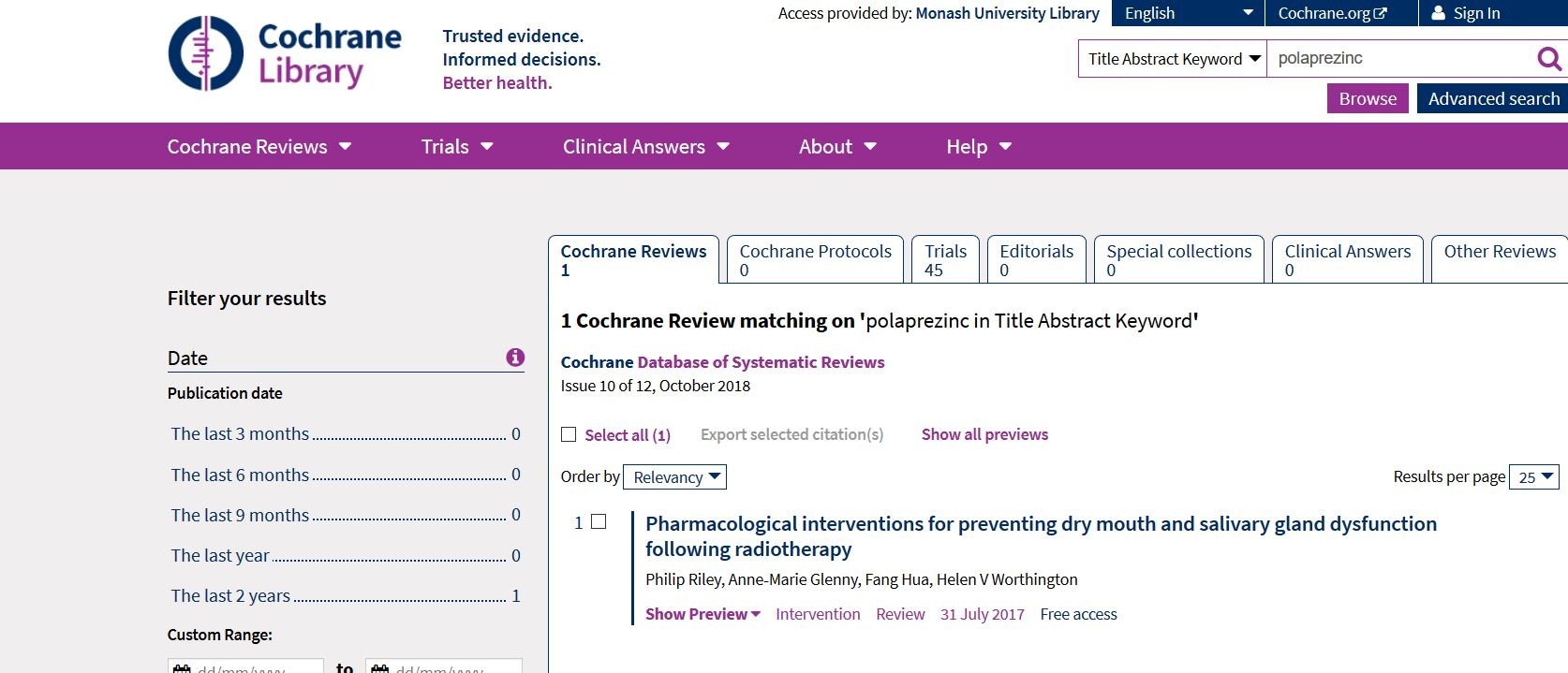
我於是用Engine查到36個entries
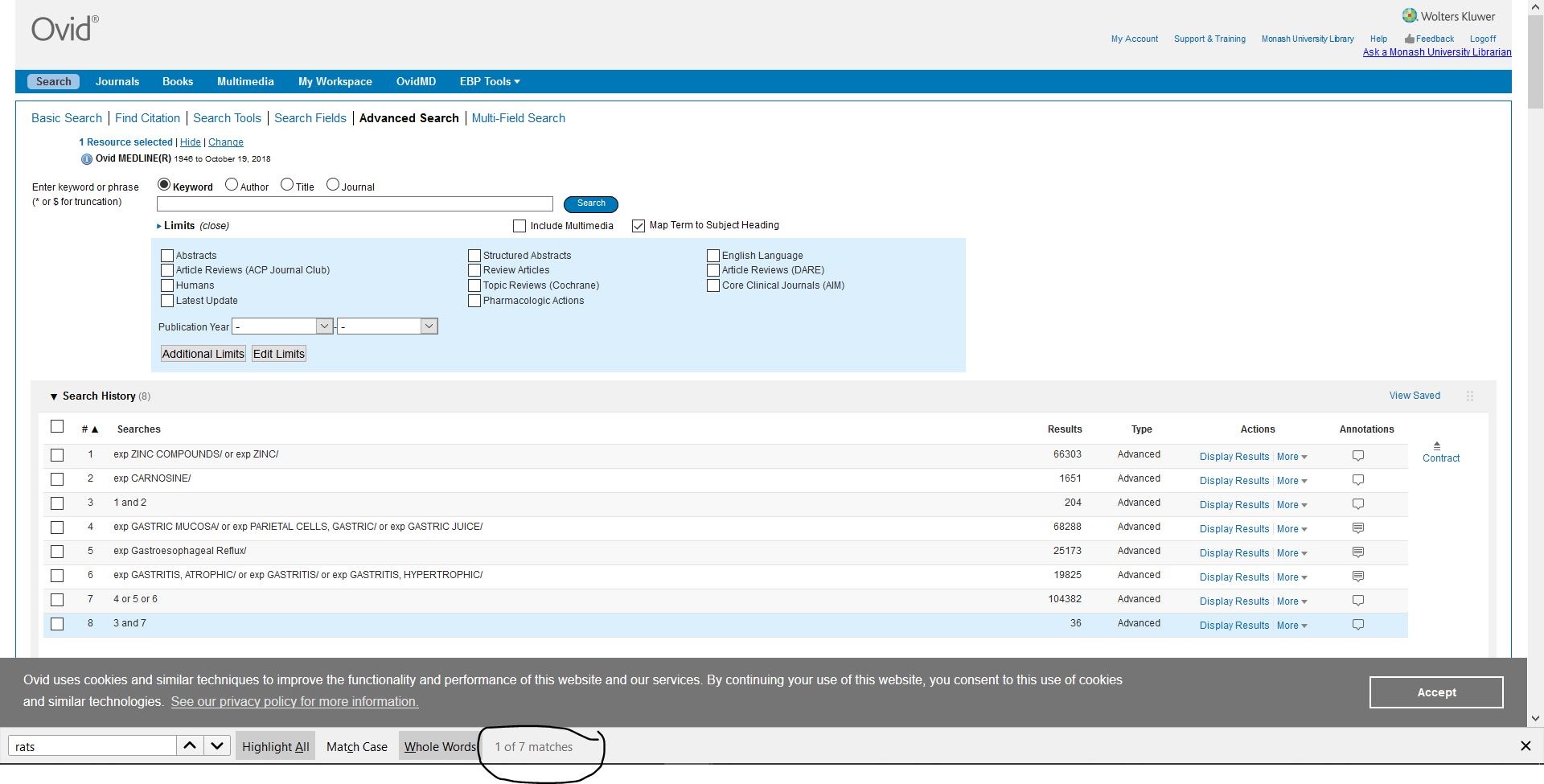
但近五年內只有2個,其中一個重要係老鼠實驗
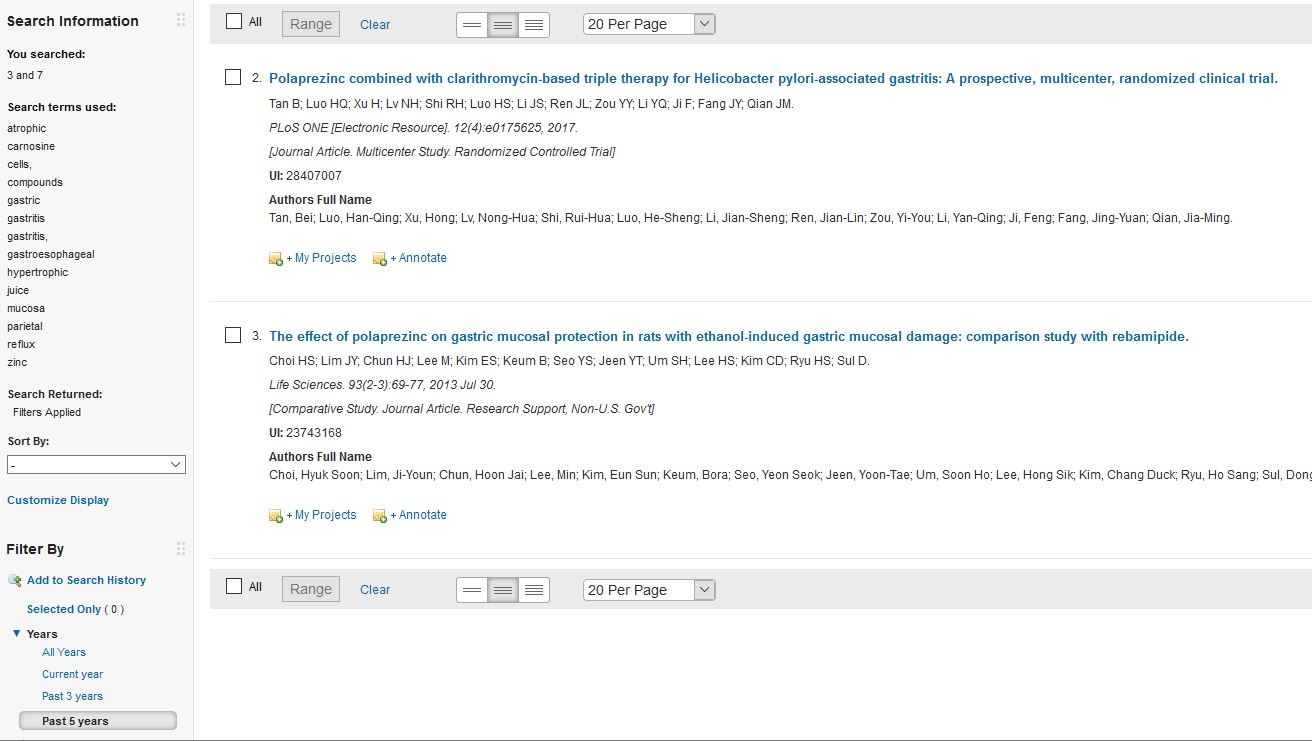
Polaprezinc combined with clarithromycin-based triple therapy for Helicobacter pylori-associated gastritis: A prospective, multicenter, randomized clinical trial:
77% 使用Triple therapy + 75mg Polaprezinc twice daily 的病患於14日內消除了H. poly
58.9% 使用Triple therapy 的病患於14日內消除了H. poly
75mg Polaprezinc vs 150mg Polaprezinc 成效差唔多,但150mg 副作用機率係高2%
原文:
H. pylori:
Helicobacter pylori (H. pylori), one of the most globally prevalent pathogens, colonizes about 50% of the world’s population. It is transmitted from human to human and causes chronic gastritis in all colonized subjects.
副作用機率:
The adverse event rate for Arm B (5.1%) was higher than for Arms A (2.8%) (P = 0.04) and C (1.9%) (P = 0.02).
篇文邊個都睇得。最好起碼睇下tables,唔明可以再問我。Table 3係講symptoms improvement, table 4 係adverse effects.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5391070/
留意返其實個sample size唔大,所以會有adverse effects佢地underestimate/無發現
南韓:
https://www-sciencedirect-com.ezproxy.lib.monash.edu.au/science/article/pii/S0016510717311884?via%3Dihub
Drug-drug interaction:
https://www.drugbank.ca/drugs/DB09221/biointeractions
加拿大視佢為實驗性質

https://www.drugbank.ca/drugs/DB09221
澳洲視佢為輔助性藥物(complementary)

https://www.tga.gov.au/compositional-guideline/polaprezinc
係我手上幾個references都無講太多
MIMs Online: No Product Information (PI) 同 Consumer Medicines Information (CMI)

Australian Medicine Handbook: Not found

Micromedex:

Cochrane Library: 只有一個entry, 但係講radiotherapy

我於是用Engine查到36個entries

但近五年內只有2個,其中一個重要係老鼠實驗

Polaprezinc combined with clarithromycin-based triple therapy for Helicobacter pylori-associated gastritis: A prospective, multicenter, randomized clinical trial:
77% 使用Triple therapy + 75mg Polaprezinc twice daily 的病患於14日內消除了H. poly
58.9% 使用Triple therapy 的病患於14日內消除了H. poly
75mg Polaprezinc vs 150mg Polaprezinc 成效差唔多,但150mg 副作用機率係高2%
原文:
H. pylori:
Helicobacter pylori (H. pylori), one of the most globally prevalent pathogens, colonizes about 50% of the world’s population. It is transmitted from human to human and causes chronic gastritis in all colonized subjects.
副作用機率:
The adverse event rate for Arm B (5.1%) was higher than for Arms A (2.8%) (P = 0.04) and C (1.9%) (P = 0.02).
篇文邊個都睇得。最好起碼睇下tables,唔明可以再問我。Table 3係講symptoms improvement, table 4 係adverse effects.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5391070/
留意返其實個sample size唔大,所以會有adverse effects佢地underestimate/無發現
南韓:
https://www-sciencedirect-com.ezproxy.lib.monash.edu.au/science/article/pii/S0016510717311884?via%3Dihub
Drug-drug interaction:
https://www.drugbank.ca/drugs/DB09221/biointeractions
功效幾大就唔知,不過個藥物原理係堅
你睇加拿大個link indication個到會有講
你睇加拿大個link indication個到會有講
Flurazepam 香港HA正當唔會開
我成日周圍走開埋開埋好多prescription 藥
我成日周圍走開埋開埋好多prescription 藥
唔知樓主仲係唔係度呢
 想問下裨乃膚同elomet cream得唔得架,我而家情況唔係咁嚴重了,冇乜點痕,不過有撻印係度
想問下裨乃膚同elomet cream得唔得架,我而家情況唔係咁嚴重了,冇乜點痕,不過有撻印係度懶得買藥膏

 想問下裨乃膚同elomet cream得唔得架,我而家情況唔係咁嚴重了,冇乜點痕,不過有撻印係度
想問下裨乃膚同elomet cream得唔得架,我而家情況唔係咁嚴重了,冇乜點痕,不過有撻印係度Take care bro !
我問左啲 HA 同行,都唔知點解  佢話有可能係啲濕勁policy
佢話有可能係啲濕勁policy
其實不停返去只係為左拎藥真係好慘 完全明係好曬時間
完全明係好曬時間 
你下次試下問下姑娘/ 出藥藥劑師點解會咁,睇下有冇得搞搞
 佢話有可能係啲濕勁policy
佢話有可能係啲濕勁policy 其實不停返去只係為左拎藥真係好慘
 完全明係好曬時間
完全明係好曬時間 
你下次試下問下姑娘/ 出藥藥劑師點解會咁,睇下有冇得搞搞

冇藥性,唔怕用得多
如果覺得唔夠力 → 搽密啲 or 換隻再"立"啲嘅
其實wet wrap 全身都做得,如果係手手腳腳你可以去藥房買啲Tubular bandage (例如 Tubigrip,利申:非打手)
用restoraderm 做潤膚膏做都OK 嘅
如果覺得唔夠力 → 搽密啲 or 換隻再"立"啲嘅
其實wet wrap 全身都做得,如果係手手腳腳你可以去藥房買啲Tubular bandage (例如 Tubigrip,利申:非打手)
用restoraderm 做潤膚膏做都OK 嘅

你食過未?
感冒止鼻水 → 如果你冇乜長期病is OK
當然休息飲水都好重要啦
食Panadol 特強食到個人好唔舒服 → 止痛特強配方 (有咖啡因)?
點唔舒服法?如果係心跳手震個啲 Clarinase 都有機會會有
感冒止鼻水 → 如果你冇乜長期病is OK
當然休息飲水都好重要啦

食Panadol 特強食到個人好唔舒服 → 止痛特強配方 (有咖啡因)?
點唔舒服法?如果係心跳手震個啲 Clarinase 都有機會會有
真係可能係gene 嘅 
可能同健身方法有關? 呢樣就真係唔識啦,要問健身教練
btw 岩search 左paul gor,好大隻wor

可能同健身方法有關? 呢樣就真係唔識啦,要問健身教練

btw 岩search 左paul gor,好大隻wor

其實藥房配藥點解一定要藥劑師?
因為我地有專業知識,可以:
確保冇配錯藥/ 確保對症下藥/ 檢查drug-drug interactions/ 教病人non-pharmacological treatments/ 解釋個病/ Ensure a proper label/ 與病人談心
你讀咁多書覺唔覺得晒左?
我唔覺,因為我覺得我係社區藥房做到嘅野係有意義的
(不代表其他藥劑師立場 )
)
因為我地有專業知識,可以:
確保冇配錯藥/ 確保對症下藥/ 檢查drug-drug interactions/ 教病人non-pharmacological treatments/ 解釋個病/ Ensure a proper label/ 與病人談心

你讀咁多書覺唔覺得晒左?
我唔覺,因為我覺得我係社區藥房做到嘅野係有意義的

(不代表其他藥劑師立場
 )
)

一陣食一粒zolpudem 先訓...
想問有冇d護肝功能好d既藥丸 → 冇
市民成日會講「補肝」,但其實係醫護人員眼中係冇呢個concept的
因為有病醫病,如果係肝病就接受治療,真係差到冇得救就換肝 (如果有得換 )
)
而且以我淺薄嘅knowledge,暫時都冇邊隻藥/ 保健品可以有效地逆轉肝臟功能,所以大家要保護好你唯一嘅肝臟呀
(有錯請指正)
所以我個人建議由生活入手啦,早啲訓,生活規律啲 AND !!!
儘量少煙酒啦 (如果有)
市民成日會講「補肝」,但其實係醫護人員眼中係冇呢個concept的
因為有病醫病,如果係肝病就接受治療,真係差到冇得救就換肝 (如果有得換
 )
)而且以我淺薄嘅knowledge,暫時都冇邊隻藥/ 保健品可以有效地逆轉肝臟功能,所以大家要保護好你唯一嘅肝臟呀

(有錯請指正)
所以我個人建議由生活入手啦,早啲訓,生活規律啲 AND !!!
儘量少煙酒啦 (如果有)
重點:避開致敏源
一般建議就係避免刺激物同致敏源,例如
- 少去花粉/ 塵多嘅地方
- 儘量少開冷氣,冷氣要定期洗 (有patient同我講佢個個星期都洗 )
)
- 唔好成日太大力"慎"個鼻
- 唔好養 貓/狗 ?
- 有d evidence 話定期做中等強度帶氧運動都可能會幫到
- 可以考慮洗鼻 (輕輕洗 or 成個鼻竇洗)
一般建議就係避免刺激物同致敏源,例如
- 少去花粉/ 塵多嘅地方
- 儘量少開冷氣,冷氣要定期洗 (有patient同我講佢個個星期都洗
 )
)- 唔好成日太大力"慎"個鼻
- 唔好養 貓/狗 ?
- 有d evidence 話定期做中等強度帶氧運動都可能會幫到
- 可以考慮洗鼻 (輕輕洗 or 成個鼻竇洗)
真係唔識sor 

濕疹有邊隻潤膚膏推介?
你用開邊隻覺得唔夠力?
因為選潤膚膏 = 如果覺得唔夠力 → 搽密啲 or 搽隻"立"啲嘅 → 用Wet wrap
好返既濕疹有黑色素沉澱可以點搞?
搽防曬等佢慢慢"登"勻返 時間會治癒一切
時間會治癒一切 
就算用去黑色素藥膏都主要係阻隔佢再整,用完有機會會一"達達" (因為附近嘅皮膚反而白左)
你用開邊隻覺得唔夠力?
因為選潤膚膏 = 如果覺得唔夠力 → 搽密啲 or 搽隻"立"啲嘅 → 用Wet wrap
好返既濕疹有黑色素沉澱可以點搞?
搽防曬等佢慢慢"登"勻返
 時間會治癒一切
時間會治癒一切 
就算用去黑色素藥膏都主要係阻隔佢再整,用完有機會會一"達達" (因為附近嘅皮膚反而白左)
我覺得冇效 OR 可以話係 效果未知
因為未有大型臨床研究數據去支持佢
而抗衰老呢家野,由秦始皇求長生到e家,似乎都唔會被人類解得開呢個謎
因為未有大型臨床研究數據去支持佢

而抗衰老呢家野,由秦始皇求長生到e家,似乎都唔會被人類解得開呢個謎

樓主尚健在 
得,但冇抗真菌效果wor (而且係較重嘅類固醇,止痕only)
如果想預防復發直接用antifungal cream 就得啦,搽多幾日咁
唔好R撻印,等佢慢慢散 時間會治癒一切
時間會治癒一切 

得,但冇抗真菌效果wor (而且係較重嘅類固醇,止痕only)
如果想預防復發直接用antifungal cream 就得啦,搽多幾日咁
唔好R撻印,等佢慢慢散
 時間會治癒一切
時間會治癒一切 
唔該樓豬
咁我暫時乜都唔搽等時間治癒佢先
估唔到個post都可以推到(就)1001
多謝咁多位巴打絲打嘅問題
我諗有d巴打見到我地user name都已經估到
其實我地係 藥劑連線 Pharmacists Connect
一班藥劑嘅後生仔 希望透過唔同嘅渠道比大家認識下我地
我諗成個post都起碼有幾百條問題 其實藥劑師嘅專業都係可以幫到大家的 (希望啦)
因為由於問題比預期多 而小弟呢排又比較忙,暫時唔得閒開 (二)
各位巴打絲打如果仲有咩健康藥物嘅問題想問可以去我地facebook inbox我地呀
fb link: https://www.facebook.com/pharmacistsconnect/
(或者直接去有藥劑師專櫃嘅社區藥房諮詢藥劑師啦!)
我地藥劑師都會繼續解答你地嘅問題
之後我地都會同大家討論下唔同嘅健康議題
如果你地有咩想知多d 都可以同我地講下
好啦 再次多謝連登巴絲幫我推post
多謝咁多位巴打絲打嘅問題
我諗有d巴打見到我地user name都已經估到
其實我地係 藥劑連線 Pharmacists Connect
一班藥劑嘅後生仔 希望透過唔同嘅渠道比大家認識下我地

我諗成個post都起碼有幾百條問題 其實藥劑師嘅專業都係可以幫到大家的 (希望啦)
因為由於問題比預期多 而小弟呢排又比較忙,暫時唔得閒開 (二)
各位巴打絲打如果仲有咩健康藥物嘅問題想問可以去我地facebook inbox我地呀

fb link: https://www.facebook.com/pharmacistsconnect/
(或者直接去有藥劑師專櫃嘅社區藥房諮詢藥劑師啦!)
我地藥劑師都會繼續解答你地嘅問題

之後我地都會同大家討論下唔同嘅健康議題
如果你地有咩想知多d 都可以同我地講下
好啦 再次多謝連登巴絲幫我推post

btw 巴打我再問左人
佢話有可能關funding 事 (eg sam fund)
如果你隻藥有資助,funding source 嘅guideline 係建議1個月拎一次藥
(可能驚特然轉藥 or 停藥 會浪費錢?)
佢話有可能關funding 事 (eg sam fund)
如果你隻藥有資助,funding source 嘅guideline 係建議1個月拎一次藥
(可能驚特然轉藥 or 停藥 會浪費錢?)