Electrolye 係一舊lithium salt in organic solvent
請問點解係organic solvent內,都會出現
Li <> Li^+ e-
CoO2 + e- <> CoO2^-
dse Chem lithium ion rechargeable cell
厹
33 回覆
1 Like
6 Dislike
有乜問題

有乜問題
正常redox如果以electrochemical series predict feasibility
條件要係aq
但而家係organic solvent, 想知點解都可以有redox
點解organic solvent 唔可以有redox??
點解organic solvent 唔可以有redox??
我無話唔得
只係想知點解姐
因為一路以來都係aq下用electrochemical series predict redox feasibility
我dse唔知organic solvent都可以有redox 姐...🙈
你好似無正面答我問題😂
你好似無正面答我問題😂
Why not 之後 ester ethene ... reaction好多係redox
唔記得D名 dse Chen
你好似無正面答我問題😂
Why not 之後 ester ethene ... reaction好多係redox
唔記得D名 dse Chen
試下放隻手落mno4-
Release electron absorb electron= redox
REDOX係可以以幾種形式表達
1. Change of charge
2. Change of hydrogen
3. Change of oxygen
4. Simply changing of oxidation number
Aqueous state 既 redox係比你參考個feasibility, 但唔代表係organic solvent唔可以有redox
例如燃燒hydrocarbon(如汽油)都係redox reaction 不過energy 唔係以電力electricity/electron形式去轉換能量
1. Change of charge
2. Change of hydrogen
3. Change of oxygen
4. Simply changing of oxidation number
Aqueous state 既 redox係比你參考個feasibility, 但唔代表係organic solvent唔可以有redox
例如燃燒hydrocarbon(如汽油)都係redox reaction 不過energy 唔係以電力electricity/electron形式去轉換能量
REDOX係可以以幾種形式表達
1. Change of charge
2. Change of hydrogen
3. Change of oxygen
4. Simply changing of oxidation number
Aqueous state 既 redox係比你參考個feasibility, 但唔代表係organic solvent唔可以有redox
例如燃燒hydrocarbon(如汽油)都係redox reaction 不過energy 唔係以電力electricity/electron形式去轉換能量
其實有咩唔係redox
 諗落好似冇
諗落好似冇REDOX係可以以幾種形式表達
1. Change of charge
2. Change of hydrogen
3. Change of oxygen
4. Simply changing of oxidation number
Aqueous state 既 redox係比你參考個feasibility, 但唔代表係organic solvent唔可以有redox
例如燃燒hydrocarbon(如汽油)都係redox reaction 不過energy 唔係以電力electricity/electron形式去轉換能量
其實有咩唔係redox諗落好似冇
Precipitation
CaCl2 + 2NaOH => Ca(OH)2 + 2NaCl
REDOX係可以以幾種形式表達
1. Change of charge
2. Change of hydrogen
3. Change of oxygen
4. Simply changing of oxidation number
Aqueous state 既 redox係比你參考個feasibility, 但唔代表係organic solvent唔可以有redox
例如燃燒hydrocarbon(如汽油)都係redox reaction 不過energy 唔係以電力electricity/electron形式去轉換能量
其實有咩唔係redox諗落好似冇
Precipitation
CaCl2 + 2NaOH => Ca(OH)2 + 2NaCl
重有冇?
應該真係無我記得
大佬呢個年代有google架喇喎
當年我無google先要記住姐
http://chemistry.stackexchange.com/questions/18492/is-there-non-redox-reaction
加油啦DSE雞們
當年我無google先要記住姐
http://chemistry.stackexchange.com/questions/18492/is-there-non-redox-reaction
加油啦DSE雞們
大佬呢個年代有google架喇喎
當年我無google先要記住姐
http://chemistry.stackexchange.com/questions/18492/is-there-non-redox-reaction
加油啦DSE雞們
Thanks
但唔同環境,個個feasibility 應該唔同?
即無organic solvent 內 feasibility (reduction potential)唔會同 aq時候一樣?
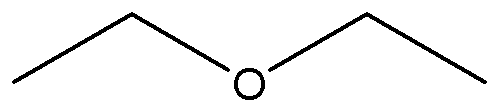
This is dry ether, an organic solvent for redox reactions.
Note the oxygen atom. You can see that it has lone pair electron for transfer.
Thanks 🐢sir
我清左最重要concept 了!
重點係有e transfer -> redox
而點有e transfer , 真係睇唔同條件下既potential differences 了...
大佬呢個年代有google架喇喎
當年我無google先要記住姐
http://chemistry.stackexchange.com/questions/18492/is-there-non-redox-reaction
加油啦DSE雞們
Thanks
但唔同環境,個個feasibility 應該唔同?
即無organic solvent 內 feasibility (reduction potential)唔會同 aq時候一樣?
aq 要睇埋 pH
Yes! I got this!
大佬呢個年代有google架喇喎
當年我無google先要記住姐
http://chemistry.stackexchange.com/questions/18492/is-there-non-redox-reaction
加油啦DSE雞們
Thanks
但唔同環境,個個feasibility 應該唔同?
即無organic solvent 內 feasibility (reduction potential)唔會同 aq時候一樣?
觀念正確
大佬呢個年代有google架喇喎
當年我無google先要記住姐
http://chemistry.stackexchange.com/questions/18492/is-there-non-redox-reaction
加油啦DSE雞們
Thanks
但唔同環境,個個feasibility 應該唔同?
即無organic solvent 內 feasibility (reduction potential)唔會同 aq時候一樣?
觀念正確
仲想問多題:corrosive property of a chemical
Dse 大概我知係
Acid / base/ alkaline metal / h2o2 / dehydrating agent
有個特別case:
AlCl3 佢都係corrosive
1. 如果我話因爲佢係acidic salt, concept上有無問題?
2. 如果無問題?是否所有acidic or alkaline salt都具有corrosive property?
大佬呢個年代有google架喇喎
當年我無google先要記住姐
http://chemistry.stackexchange.com/questions/18492/is-there-non-redox-reaction
加油啦DSE雞們
Thanks
但唔同環境,個個feasibility 應該唔同?
即無organic solvent 內 feasibility (reduction potential)唔會同 aq時候一樣?
觀念正確
仲想問多題:corrosive property of a chemical
Dse 大概我知係
Acid / base/ alkaline metal / h2o2 / dehydrating agent
有個特別case:
AlCl3 佢都係corrosive
1. 如果我話因爲佢係acidic salt, concept上有無問題?
2. 如果無問題?是否所有acidic or alkaline salt都具有corrosive property?
AlCl3 + HCl ---> H(AlCl4)
AlCl3 is actually not purely acidic.
Anyway, a dissolved chemical that can change pH from neutral is corrosive by definition.
了解des
AlCl3 + HCl ---> H(AlCl4)
Hcl本身就acidic 喎
Alcl3 is a strong Lewis acid,
Which can react with water to form hydronium ion
[Al(H2O)6]3+ ⇌ [Al(OH)(H2O)5]2+ + H+
Hcl本身就acidic 喎
Alcl3 is a strong Lewis acid,
Which can react with water to form hydronium ion
[Al(H2O)6]3+ ⇌ [Al(OH)(H2O)5]2+ + H+