[DSE化學2019]同學準備好DSE CHEM未? (2)
化學艾維斯
995 回覆
21 Like
2 Dislike
基本要記methyl orange同phenolphthalein幾時用
dipole moment out 左 c, 如果係asymmetrical molecule 例如 NH3 解釋佢係polar, 之前會話N is more electronegative than H, N-H bond is polar, the molecule is asymmetrical and the dipole moments cannot cancel out each other, therefore the molecule is polar咁啦,如果我寫dipole moment 係咪唔比分,如果唔比,要改咩呀,我見有d書就咁改dipole moment 做polarity 係咪咁

同理 cis-ch2cl2 個boiling point 大過trans點解釋,dipole moment out 左c



同理 cis-ch2cl2 個boiling point 大過trans點解釋,dipole moment out 左c


搞錯左d野c2h2cl2至啱



唔會唔比分 事實係咁
其實我都建議寫返dipole moment 無謂夾硬搵第二個字取代佢
cis trans咪一樣用返asymmetrical > net dipole 去解
其實我都建議寫返dipole moment 無謂夾硬搵第二個字取代佢
cis trans咪一樣用返asymmetrical > net dipole 去解
原來之前睇d片都係巴打拍,唔該曬巴打



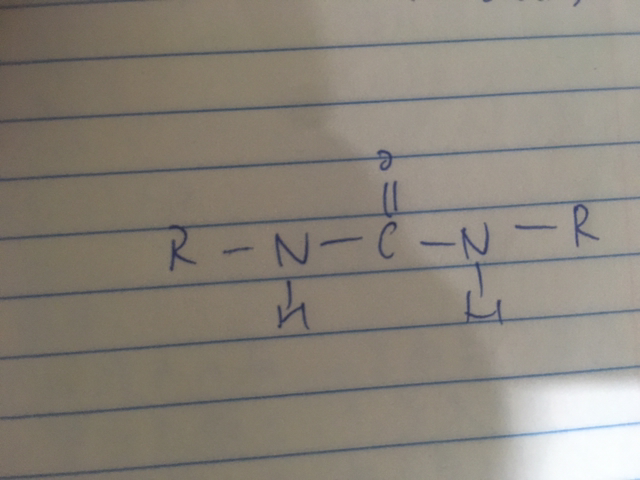
咁樣應該計一個amide group定兩個amide group?
多謝你支持就真

urea係另一個series黎 dse無正式學的
2018 Paper2 infra-red spectrum C=C個absorption peak點睇到出黎?
同埋distinguish K2SO3 and K2SO4 用acidified KMnO4可唔可以?
同埋distinguish K2SO3 and K2SO4 用acidified KMnO4可唔可以?
C=C係好難睇 通常係估structure果時發現少左兩個H去推斷返
KMnO4 ok
KMnO4 ok
different substance有different solubility in stationary phase and mobile phase
ok 唔該曬~
當然
隨你放咩solvent落去
用唔同solvent, 最後就會有不同既Rf value
隨你放咩solvent落去
用唔同solvent, 最後就會有不同既Rf value
giant molecular淨係識c=c整出黎既polymer就得
定係要識埋general properties
定係要識埋general properties
HPLC洗唔洗考?同埋mohr method 洗唔洗要自己知曬成個流程然後直接問你
另外想問點解co2+ [Al(OH)4]-會出Al(OH)3?
sulphurous acid 係 weak 定strong acid?
我睇acidic/alkaline hydrolysis of amide/ester 係organic chem 都要heat
但rate reaction 個課ester+naoh 唔使heat 都行到alkaline hydrolysis 既product 係咪其實acidic/alkaline hydrolysis of amide/ester 唔使heat 都行到,heat 個功能只係for faster the reaction?

我睇acidic/alkaline hydrolysis of amide/ester 係organic chem 都要heat
但rate reaction 個課ester+naoh 唔使heat 都行到alkaline hydrolysis 既product 係咪其實acidic/alkaline hydrolysis of amide/ester 唔使heat 都行到,heat 個功能只係for faster the reaction?


唔考
佢會比資料你
佢會比資料你
CO2弱酸性
會慢慢拉低pH咁咪shift eqm LHS
會慢慢拉低pH咁咪shift eqm LHS
weak架
yes唔heat會好慢
synthesis要寫明heat
yes唔heat會好慢
synthesis要寫明heat
重有electrolysis 如果cu 係anode, electrolyte 係cuso4(aq), Cu->Cu2+ +2e- , 但同時 Cu2+ +2e- ->Cu, 個effect cancel out左, Cu metal 個mass remains unchanged
有年past paper chemical cell 係mg in mgso4 solution 做 reducing agent, 因為mg 係strong reducing agent, 佢loses electrons, 之後出mg2+但mg2+ hardly reduced 所以唔會變返mg, mg dissolves.
我想問如果zn anode in znso4(aq), al anode in al2(so4)3係咪同mg 一樣都行唔到
zn2+ +2e- -> zn
al3+ +2e- -> al
zn 同al 都會dissolves and decrease in mass


有年past paper chemical cell 係mg in mgso4 solution 做 reducing agent, 因為mg 係strong reducing agent, 佢loses electrons, 之後出mg2+但mg2+ hardly reduced 所以唔會變返mg, mg dissolves.
我想問如果zn anode in znso4(aq), al anode in al2(so4)3係咪同mg 一樣都行唔到
zn2+ +2e- -> zn
al3+ +2e- -> al
zn 同al 都會dissolves and decrease in mass


